Governor Carney, DPH, Bayhealth Announce First Delawarean Has Received the COVID-19 Vaccine
Governor John Carney | Office of the Governor | Date Posted: Tuesday, December 15, 2020
Governor John Carney | Office of the Governor | Date Posted: Tuesday, December 15, 2020
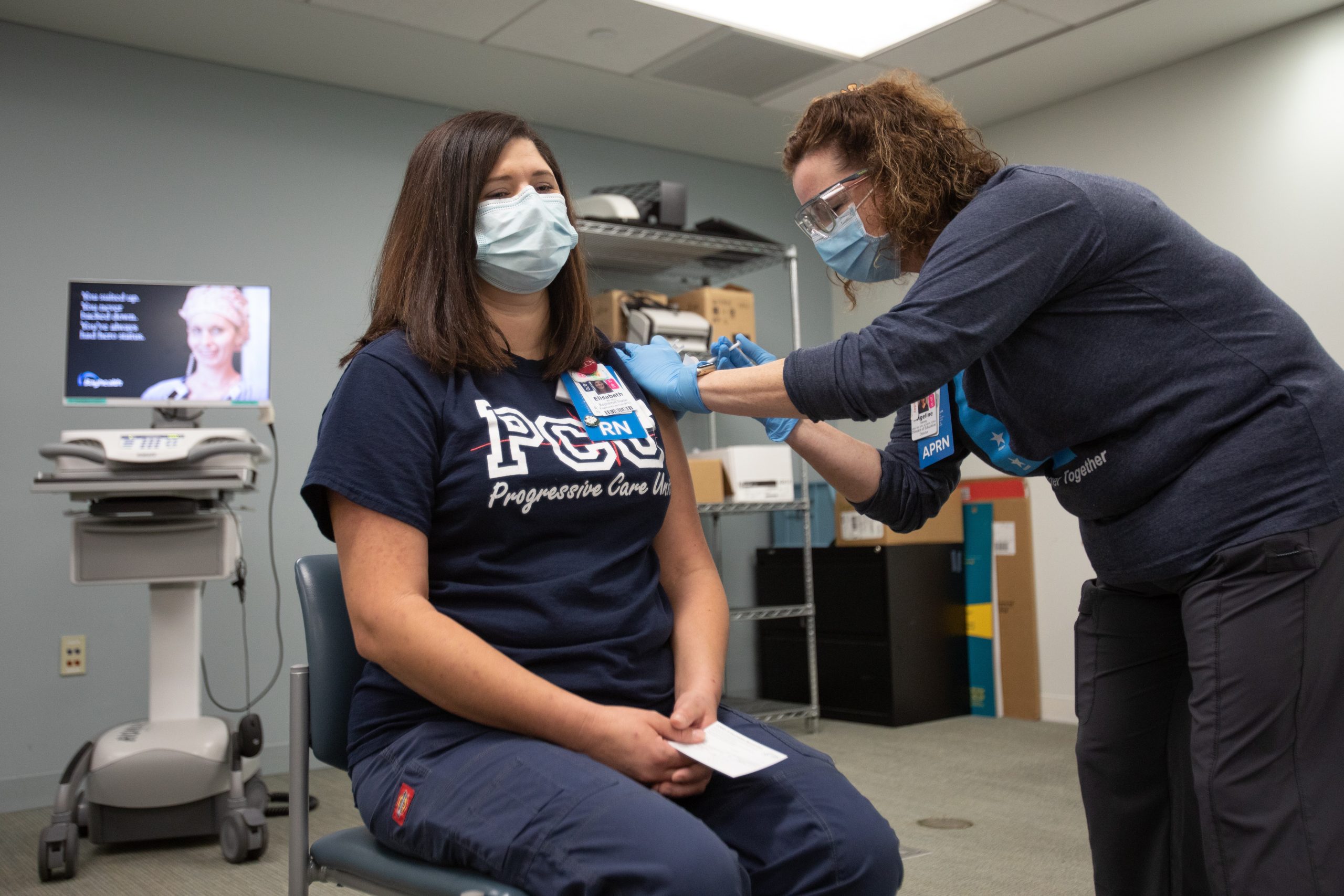
DOVER, Del. – Governor John Carney, the Delaware Division of Public Health (DPH) and Bayhealth announced that the first Delawarean received the Pfizer BioNTech COVID-19 vaccine on Tuesday morning at Bayhealth’s Kent campus in Dover.
Elisabeth Cote, a progressive care unit nurse at Bayhealth, received the vaccine roughly 24 hours after the first shipment arrived at Bayhealth on Monday. Cote is part of the Phase 1a group of frontline health care staff who are slated to receive the vaccine first.
“After nine long months fighting COVID-19, this is a moment of hope for Delaware and for our country. There is a light at the end of the tunnel,” said Governor Carney. “Delaware’s nurses, doctors, nursing assistants, and non-medical staff have all demonstrated courage and leadership every day of this COVID-19 crisis. Now, nurses like Elisabeth Cote are leading by example again by stepping up to get the COVID-19 vaccine. It is because of them that we will beat this pandemic and come out stronger on the other side.”
Bayhealth is the first health care system in Delaware to receive the vaccine.
“These vaccines will provide critical protection to our frontline workers whose lives are at risk every day due to COVID-19,” said Bayhealth President and CEO Terry M. Murphy, FACHE. “I’m proud of how our team at Bayhealth has responded to this pandemic, especially as we take this first step to protect our entire community with the help of this vaccine in the months ahead.”
Delaware, which received 975 of its 8,775 pre-ordered doses from Pfizer, was one of the first states in the nation to receive initial doses of vaccine on Monday. The Delaware Division of Public Health (DPH), which is coordinating the state’s efforts to acquire and distribute the vaccine, expects to receive the remainder of the vaccine doses at its Kent County warehouse on Wednesday.
The Pfizer vaccine is required to be kept at below-freezing temperatures. After receipt, DPH will store the doses it receives on Wednesday in its ultra-cold storage unit and will begin scheduling delivery to the remainder of the state’s health care systems. Those doses will be distributed within 24 to 48 hours after the shipment is received.
DPH, which is responsible for providing the framework for acquiring and distributing the vaccine, has devised a three-tier strategy for distribution.
While DPH does not plan to mandate the vaccine, it is strongly encouraging that people, particularly healthcare workers, get vaccinated once doses become available.
The Pfizer BioNTech COVID-19 vaccine was granted Emergency Use Authorization by the U.S. Food & Drug Administration last week.
Children under the age of 16 are not included in the initial three phases of the vaccine’s rollout, as the FDA has not yet approved its use for individuals who fall into this category. More clinical trials involving children under 16 are still needed.
The FDA and the Centers for Disease Control and Prevention (CDC) are advising women who are pregnant or breastfeeding, individuals who have experienced allergic reactions to other vaccines and those who have compromised immune systems should discuss the benefits and risks of taking the vaccine with their medical provider before receiving it.
The potential side effects from the vaccine are similar to those experienced by people who receive the flu shot: soreness at the injection site, fever, headaches, and body aches that usually go away within 24 hours. Unless symptoms worsen or linger, there is no need to seek medical care. Pfizer reported no serious side effects from the vaccine, and there were no deaths directly linked to the vaccine itself. The FDA and CDC will continue to monitor the COVID-19 vaccine for safety and effectiveness and any long-term or rare side effects.
The Pfizer vaccine has a 90 percent effectiveness rate. Comparatively, the flu vaccine is generally 40 to 60 percent effective. The COVID-19 vaccine does not contain a live virus and cannot give individuals the coronavirus.
The Pfizer vaccine does require two doses spaced about three weeks a part to be effective. The same brand of vaccine must be administered for both doses. DPH plans to remind individuals to get their second dose of the vaccine by sending reminder letters, providing automated phone calls and text messages and by patient record cards.
DPH is in the process of setting up a Vaccine Call Center, which it expects to be operational soon. Individuals can email their questions concerning the vaccine to Vaccine@delaware.gov. Individuals can also visit de.gov/covidvaccine for up-to-date information. Myhealthycommunity.dhss.delaware.gov will also have data on the vaccine available.
###
Related Topics: Coronavirus, COVID-19 Vaccine, governor, Governor Carney
Keep up to date by receiving a daily digest email, around noon, of current news release posts from state agencies on news.delaware.gov.
Here you can subscribe to future news updates.
Governor John Carney | Office of the Governor | Date Posted: Tuesday, December 15, 2020

DOVER, Del. – Governor John Carney, the Delaware Division of Public Health (DPH) and Bayhealth announced that the first Delawarean received the Pfizer BioNTech COVID-19 vaccine on Tuesday morning at Bayhealth’s Kent campus in Dover.
Elisabeth Cote, a progressive care unit nurse at Bayhealth, received the vaccine roughly 24 hours after the first shipment arrived at Bayhealth on Monday. Cote is part of the Phase 1a group of frontline health care staff who are slated to receive the vaccine first.
“After nine long months fighting COVID-19, this is a moment of hope for Delaware and for our country. There is a light at the end of the tunnel,” said Governor Carney. “Delaware’s nurses, doctors, nursing assistants, and non-medical staff have all demonstrated courage and leadership every day of this COVID-19 crisis. Now, nurses like Elisabeth Cote are leading by example again by stepping up to get the COVID-19 vaccine. It is because of them that we will beat this pandemic and come out stronger on the other side.”
Bayhealth is the first health care system in Delaware to receive the vaccine.
“These vaccines will provide critical protection to our frontline workers whose lives are at risk every day due to COVID-19,” said Bayhealth President and CEO Terry M. Murphy, FACHE. “I’m proud of how our team at Bayhealth has responded to this pandemic, especially as we take this first step to protect our entire community with the help of this vaccine in the months ahead.”
Delaware, which received 975 of its 8,775 pre-ordered doses from Pfizer, was one of the first states in the nation to receive initial doses of vaccine on Monday. The Delaware Division of Public Health (DPH), which is coordinating the state’s efforts to acquire and distribute the vaccine, expects to receive the remainder of the vaccine doses at its Kent County warehouse on Wednesday.
The Pfizer vaccine is required to be kept at below-freezing temperatures. After receipt, DPH will store the doses it receives on Wednesday in its ultra-cold storage unit and will begin scheduling delivery to the remainder of the state’s health care systems. Those doses will be distributed within 24 to 48 hours after the shipment is received.
DPH, which is responsible for providing the framework for acquiring and distributing the vaccine, has devised a three-tier strategy for distribution.
While DPH does not plan to mandate the vaccine, it is strongly encouraging that people, particularly healthcare workers, get vaccinated once doses become available.
The Pfizer BioNTech COVID-19 vaccine was granted Emergency Use Authorization by the U.S. Food & Drug Administration last week.
Children under the age of 16 are not included in the initial three phases of the vaccine’s rollout, as the FDA has not yet approved its use for individuals who fall into this category. More clinical trials involving children under 16 are still needed.
The FDA and the Centers for Disease Control and Prevention (CDC) are advising women who are pregnant or breastfeeding, individuals who have experienced allergic reactions to other vaccines and those who have compromised immune systems should discuss the benefits and risks of taking the vaccine with their medical provider before receiving it.
The potential side effects from the vaccine are similar to those experienced by people who receive the flu shot: soreness at the injection site, fever, headaches, and body aches that usually go away within 24 hours. Unless symptoms worsen or linger, there is no need to seek medical care. Pfizer reported no serious side effects from the vaccine, and there were no deaths directly linked to the vaccine itself. The FDA and CDC will continue to monitor the COVID-19 vaccine for safety and effectiveness and any long-term or rare side effects.
The Pfizer vaccine has a 90 percent effectiveness rate. Comparatively, the flu vaccine is generally 40 to 60 percent effective. The COVID-19 vaccine does not contain a live virus and cannot give individuals the coronavirus.
The Pfizer vaccine does require two doses spaced about three weeks a part to be effective. The same brand of vaccine must be administered for both doses. DPH plans to remind individuals to get their second dose of the vaccine by sending reminder letters, providing automated phone calls and text messages and by patient record cards.
DPH is in the process of setting up a Vaccine Call Center, which it expects to be operational soon. Individuals can email their questions concerning the vaccine to Vaccine@delaware.gov. Individuals can also visit de.gov/covidvaccine for up-to-date information. Myhealthycommunity.dhss.delaware.gov will also have data on the vaccine available.
###
Related Topics: Coronavirus, COVID-19 Vaccine, governor, Governor Carney
Keep up to date by receiving a daily digest email, around noon, of current news release posts from state agencies on news.delaware.gov.
Here you can subscribe to future news updates.